Registry Features
Data collection
Eurocrine collects data on surgically treated tumours within the diagnoses groups of thyroid, primary and secondary hyperparathyroidism, adrenal, paraganglioma and GEP-NET tumours.
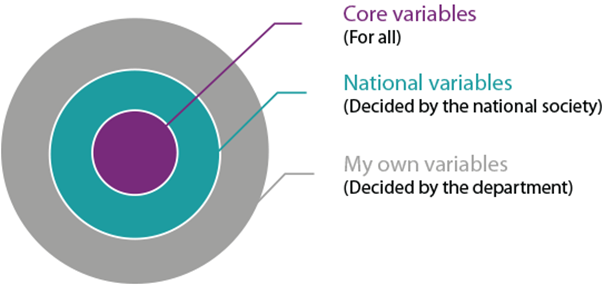
A central set of variables mandatory for all participants ensures data congruity. An additional data set is added at the national level. In addition, it is possible for a clinic to define and add their own local variables, and also share this dataset with other clinics.
Globally stored data
The global application handles most of the logic and the collected data, but do not handle the patient’s personal identification. All collected data is stored in the global application on a pseudo-ID representing the patient and operation.
Locally stored data
Information concerning patient personal data and mapping between Patient-ID and pseudo-ID is stored and kept locally at the participating hospital and clinic.
My Eurocrine
The unique module My Eurocrine has dual purposes; to enable a clinic or hospital to define and collect their own data and to perform clinical studies.
With this feature you can create your own variables for collection of data, perform observational studies, and randomised controlled trials (RCT: s).
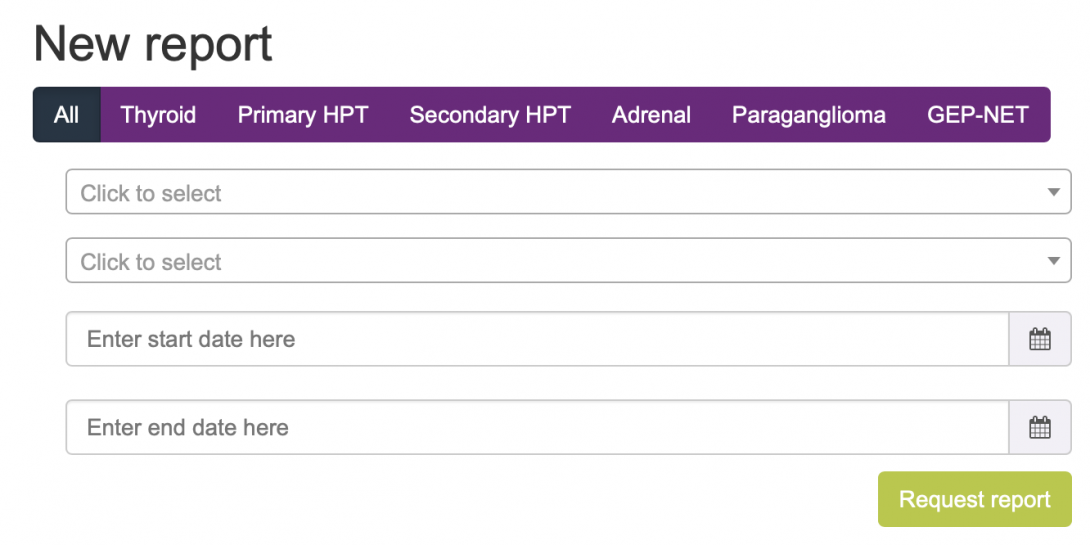
Output
Data output is available in three formats:
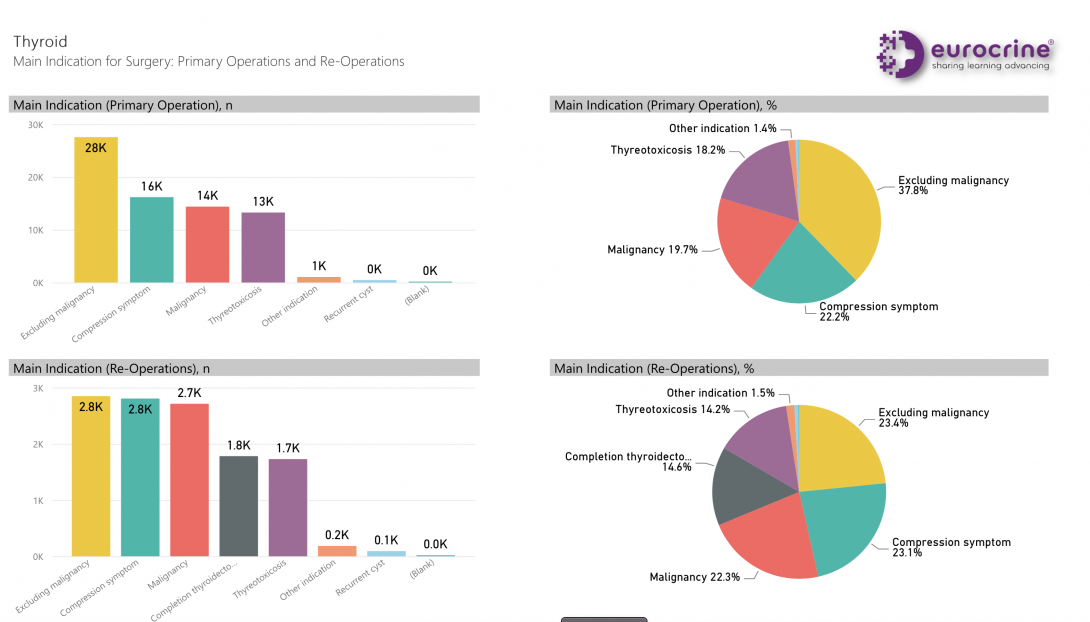
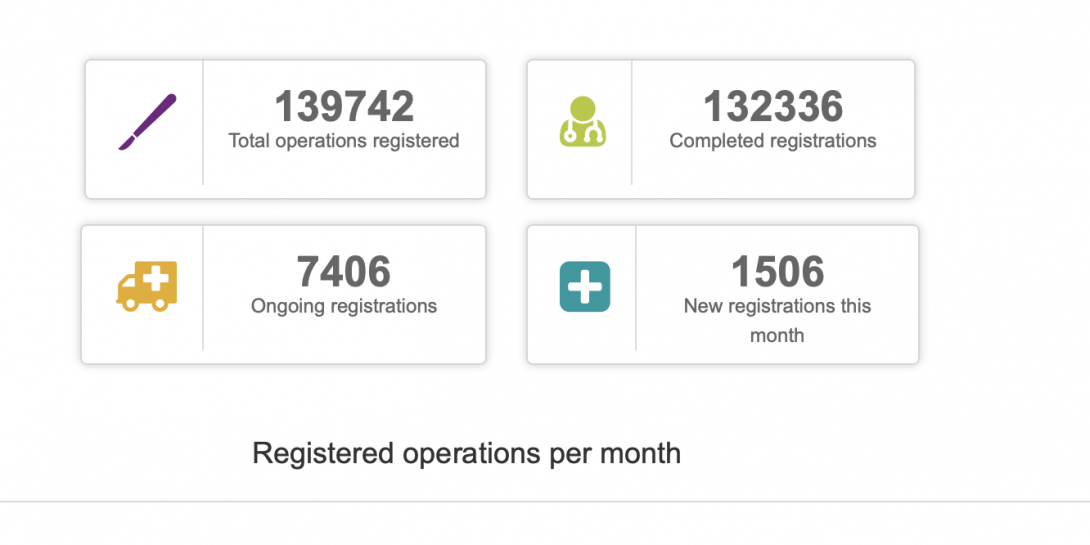
• Dashboard on registered data and generic statistics on display inside the registry, as shown in the picture below.
• All data entries in the Eurocrine are accessed directly by the user in Excel format.
Microsoft Power BI
Data collected in Eurocrine is uploaded and available in Microsoft Power BI, with access for local and national representatives. Annual standard reports are provided for Eurocrine.